
Michael Lazarus, PhD
About Me
Our lab is looking at how cells respond to nutrient changes and how we can exploit these mechanisms in human diseases including cancer and diabetes. Our approach is a multidisciplinary one that uses biochemistry, x-ray crystallography, and pharmacology to study these systems.
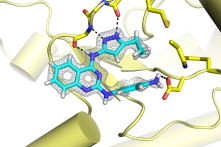 We are interested in two main areas that respond to nutrient status. The first is a pathway called autophagy, a conserved process whereby cellular components are degraded for energy, building blocks, and quality control. We are interested in developing tools to modulate autophagy in cells, and in evaluating autophagy inhibition as a therapeutic strategy for cancer. We have developed small molecule inhibitors of kinases that control this pathway.
We are interested in two main areas that respond to nutrient status. The first is a pathway called autophagy, a conserved process whereby cellular components are degraded for energy, building blocks, and quality control. We are interested in developing tools to modulate autophagy in cells, and in evaluating autophagy inhibition as a therapeutic strategy for cancer. We have developed small molecule inhibitors of kinases that control this pathway.
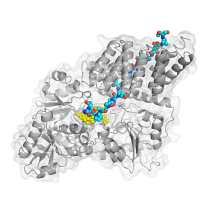 The second area we are focused on is the unusual nutrient-sensing glycosyltransferase O-GlcNAc Transferase (OGT). This enzyme responds to levels of glucose and other nutrients by dynamically glycosylating cellular signaling components and epigenetic modulating enzymes. This single enzyme glycosylates over 1,000 nucleocytoplasmic substrates through unknown mechanisms. We are interested in using biochemical and crystallographic approaches to understand the mechanisms that link glucose levels and epigenetic regulation.
The second area we are focused on is the unusual nutrient-sensing glycosyltransferase O-GlcNAc Transferase (OGT). This enzyme responds to levels of glucose and other nutrients by dynamically glycosylating cellular signaling components and epigenetic modulating enzymes. This single enzyme glycosylates over 1,000 nucleocytoplasmic substrates through unknown mechanisms. We are interested in using biochemical and crystallographic approaches to understand the mechanisms that link glucose levels and epigenetic regulation.
Language
English
Position
ASSOCIATE PROFESSOR | Graduate Education, ASSOCIATE PROFESSOR | Pharmacological Sciences
Research Topics
Biochemistry, Cancer, Drug Design and Discovery, Neuro-degeneration/protection, Structural Biology
Multi-Disciplinary Training Areas
Cancer Biology [CAB], Disease Mechanisms and Therapeutics (DMT)
Education
BS, Yale University
PhD, Harvard University
Awards
2020
Irma T. Hirschl Career Scientist Award
2017
R35 Maximizing Investigators’ Research Award
NIH
2016
Mallinckrodt Foundation Grant Award
2016
K22 Transition Career Award
NIH
2013
Helen Hay Whitney Foundation Postdoctoral Fellowship
Publications
Selected Publications
- Correction to “Characterization, Structure, and Inhibition of the Human Succinyl-CoA:glutarate-CoA Transferase, a Putative Genetic Modifier of Glutaric Aciduria Type 1”. Ruoxi Wu, Susmita Khamrui, Tetyana Dodatko, João Leandro, Amanda Sabovic, Sara Violante, Justin R. Cross, Eric Marsan, Kunal Kumar, Robert J. DeVita, Michael B. Lazarus, Sander M. Houten. ACS Chemical Biology
- Structural insights into RNA cleavage by a novel family of bacterial RNases. Ruoxi Wu, Shakti Ingle, Sarah A. Barnes, Heather R. Dahlin, Susmita Khamrui, Yufei Xiang, Yi Shi, David H. Bechhofer, Michael B. Lazarus. Nucleic Acids Research
- Characterization, Structure, and Inhibition of the Human Succinyl-CoA:glutarate-CoA Transferase, a Putative Genetic Modifier of Glutaric Aciduria Type 1. Ruoxi Wu, Susmita Khamrui, Tetyana Dodatko, Joaõ Leandro, Amanda Sabovic, Sara Violante, Justin R. Cross, Eric Marsan, Kunal Kumar, Robert J. DeVita, Michael B. Lazarus, Sander M. Houten. ACS Chemical Biology